Principles of Ring-Opening Polymerization
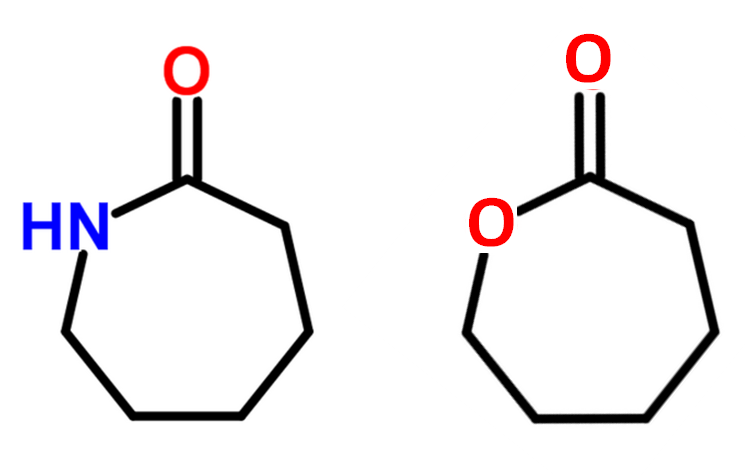
A ring-opening polymerization (ROP) is another form of chain-growth polymerization in which the terminal end group of a polymer chain acts as a reactive center where further cyclic monomers can be added by ring-opening and additon of the broken bond. Typical monomers that can be polymerized via ROP are di-functional monomers that carry two different reactive groups like one amine or alcohol and one carboxylic acid that have undergone a cyclization reaction. Two examples are caprolactam and caprolactone:
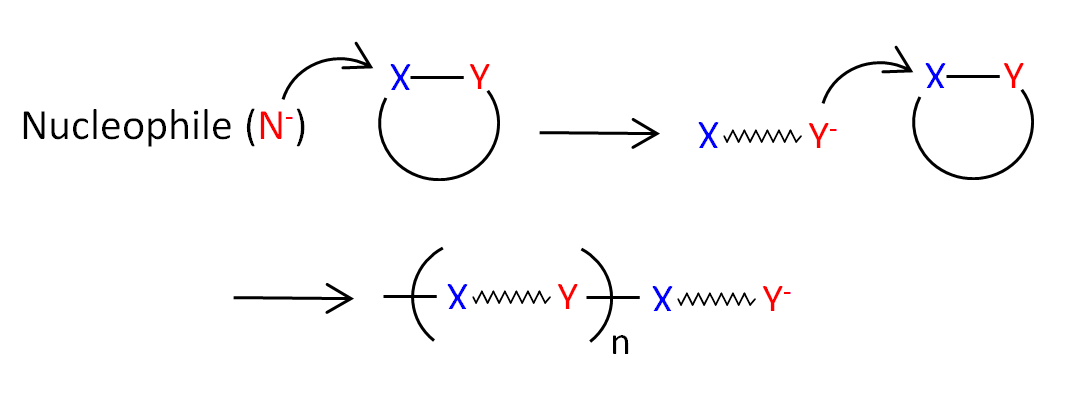
To polymerize these moieties, one of the rings has to open prior polymerization. This can be achieved, for example, by adding a small amount of a nucleophilic reagent (Lewis base) as an initiator. This reaction is called anionic ring-opening polymerization (AROP):
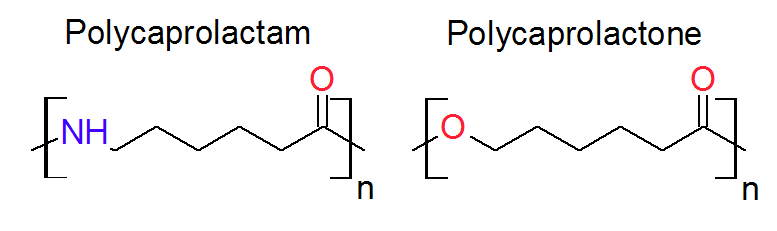
Two well known thermoplastic polymers that can be synthesized via anionic ring-opening polymerization are polycaprolactam (Nylon 6) and polycaprolactone (PCL):
Most monomers that undergo AROP contain polar bonds like ester, amide, carbonate, urethane, epoxide, and phosphate which polymerize to the corresponding polyester, polyamide, polycarbonate, polyurethane, polyepoxide, and polyphosphate.
Cationic ring-opening polymerization (CROP) is also possible. In this case, a small amount of an electrophilic reagent (Lewis acid) is added to the monomer to initiate polymerization. However, not all cyclic monomers containing a heteroatom undergo CROP.
Whether and how readily a cyclic monomer undergoes ROP depends on the ring size, to be more specific, on the ring strain which is caused by bond angle distortion (angular strain), repulsion between eclipsed hydrogen atoms (conformational strain) and non-bonding interactions between substituents attached to different parts of the ring (transannular strain).1 Small cyclic monomers with large ring strain such 3-, 4-, and 5-membered rings of cyclic esters and amides polymerize readily through ROP whereas 7- and 8-membered lactones and lactams are less reactive due to their much lower ring strain (ca. 6 J/mol) but are still reactive enough to undergo ROP.2,3 An entropy gain due to additional degrees of rotation after ring opening can be an additional driving force, as it is the case with cyclic carbonates.
Some examples of cyclic monomers that polymerize through anionic or cationic ring-opening polymerization include cyclic ethers, lactones, lactams, carbonates, aziridines, and epoxides.
Ring-opening polymerization can also proceed via free radical polymerization.4 The introduction of an oxygen into the ring will usually promote free radical ring-opening polymerization, because the resulting carbon-oxygen double bond after ring-opening is much more stable than a carbon-carbon double bond. Thus, cyclic hetero monomers with an oxygen in the ring structure and a vinyl side group like cyclic ketene acetals, cyclic ketene aminals, cyclic vinyl ethers, and unsaturated spiro ortho esters will readily undergo free radical ring-opening polymerization.6,7 Copolymerization of these monomers with a wide variety of vinyl monomers will introduce ester, amide, keto or carbonate groups into the backbone, which results in functionally terminated oligomers.
Notes & References:
1W. Saiyasombata, R. Molloya, T.M. Nicholson, A.F. Johnson, I.M. Ward, S. Poshyachinda, Polymer 39, 23, 5581-5585 (1998)
2O. Nuyken 1 and S.D. Pask, Polymers 5, 361-403 (2013)
3The ring strain of 3-, 4-, and 5-membered lactones is about 116, 111, and 27.5 kJ/mol, respectively.8
4Free radical ring-opening polymerization are not very common. In fact most five- and six-membered carboxylic structures are more prone to undergo free radical ring-closing. Some exceptions are cyclopropane derivatives such as vinylcyclopropane and highly strained bi-cyclic olefins.5
5W.J. Bailey et al., J. Macromol. Sci.: Part A - Chem. Vol. 21, 13-14 (1984)
6W. J. Bailey, Polymer Journal, 17, 1, 85 - 95 (1985)
7A carbonyl bond is approximately 50 kcal more stable than a carbon-carbon double bond.6
8E.L. Eliel, Stereochemistry of Carbon Compounds, McGraw-Hill, New York (1962)
Revised September 12, 2019